Sacrificed dog remains feed tales of Bronze Age ‘wolf-men’ warriors
Remains of at least two Late Bronze Age initiation ceremonies, in which teenage boys became warriors by eating dogs and wolves, have turned up in southwestern Russia, two archaeologists say. The controversial finds, which date to between roughly 3,900 and 3,700 years ago, may provide the first archaeological evidence of adolescent male war bands described in ancient texts.
Select boys of the Srubnaya, or Timber Grave, culture joined youth war bands in winter rites, where they symbolically became dogs and wolves by consuming canine flesh, contend David Anthony and Dorcas Brown, both of Hartwick College in Oneonta, N.Y. This type of initiation ceremony coincides with myths recorded in texts from as early as roughly 2,000 years ago by speakers of Indo-European languages across Eurasia, the researchers report in the December Journal of Anthropological Archaeology.
Those myths link dogs and wolves to youthful male war bands, warfare and death. In the ancient accounts, young warriors assumed names containing words for dogs or wolves, wore dog or wolf skins and, in some cases, ate dogs during initiation ceremonies.
Mythic themes involving dogs from 2,000 years ago may differ from the rites practiced 4,000 years ago, Anthony acknowledges. “But we should look at myths across Eurasia to understand this archaeological site,” he says.
But some researchers are unconvinced by the pair’s explanation for why at least 64 dogs and wolves were sacrificed at the Krasnosamarskoe settlement.
“Archaeologists can weave mythology and prehistory together, but only with extreme caution,” says archaeologist Marc Vander Linden of University College London.
At most, Indo-European mythology suggests that Late Bronze Age folks regarded dogs as having magical properties and perhaps ate them in rituals of some kind, Vander Linden says. But no other archaeological sites have yielded evidence for teenage male war bands or canine-consuming initiation rites, raising doubts about Anthony and Brown’s proposed scenario, he argues.
Some ancient Indo-European myths attribute healing powers to dogs, says archaeologist Paul Garwood of the University of Birmingham in England. In those myths, dogs absorb illness from people, making the canines unfit for consumption. Perhaps ritual specialists at Krasnosamarskoe sacrificed dogs and wolves as part of healing ceremonies without eating the animals, Garwood proposes.
Dog and wolf deposits at the Russian site align with myths connecting these animals to war bands and initiation rites, not healing, Anthony responds.
Michael Witzel, an authority on ancient texts of India and comparative mythology at Harvard University, agrees. Anthony and Brown have identified the first archaeological evidence in support of ancient Indo-European myths about young, warlike “wolf-men” who lived outside of society’s laws, he says.
Excavations at Krasnosamarskoe in 1999 and 2001 yielded 2,770 dog bones, 18 wolf bones and six more bones that came from either dogs or wolves. Those finds represent 36 percent of all animal bones unearthed at the site. Dogs account for no more than 3 percent of animal bones previously unearthed at each of six other Srubnaya settlements, so canines were not typically eaten and may have been viewed as a taboo food under most circumstances, the investigators say.
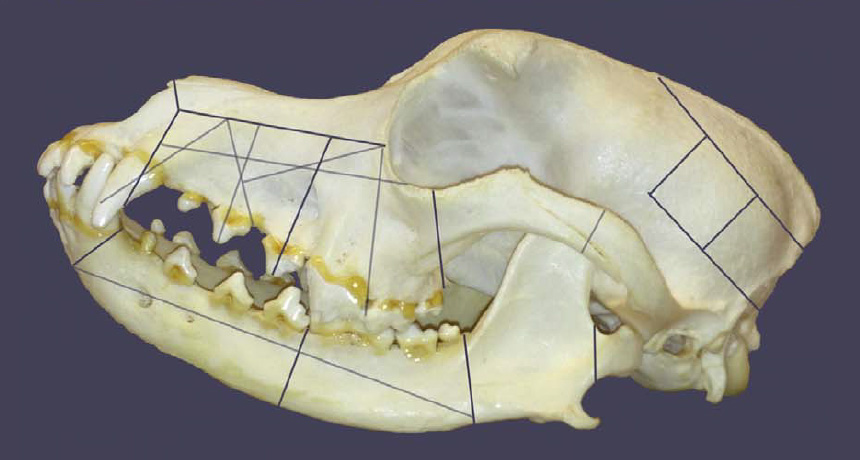
Bones from dogs’ entire bodies displayed butchery marks and burned areas produced by roasting. Dogs’ heads were chopped into 3- to 7-centimeter-wide pieces using a standardized sequence of cuts. It was a brutal, ritual behavior that demanded practice and skill, Anthony asserts. Cattle and sheep or goat remains at Krasnosamarskoe also show signs of butchery and cooking but do not include any sliced-and-diced skulls.
Separate arrays of dog bones indicate that at least two initiation ceremonies, and possibly several more, occurred over Krasnosamarskoe’s 200-year history. Microscopic analyses of annual tissue layers in tooth roots of excavated animals indicated that dogs almost always had been killed in the cold half of the year, from late fall through winter. Cattle were slaughtered in all seasons, so starvation can’t explain why dogs were sometimes killed and eaten, the researchers say.
DNA extracted from teeth of 21 dogs tagged 15 as definitely male and another four as possibly male, leaving two confirmed females. A focus on sacrificing male dogs at Krasnosamarskoe is consistent with a rite of passage for young men, Anthony says.
Excavations of a Srubnaya cemetery at the Russian site produced bones of two men, two women, an adult of undetermined sex and 22 children, most between ages 1 and 7. The two men, who both displayed injuries from activities that had put intense stress on their knees, ankles and lower backs, may have been ritual specialists, the researchers speculate. These men would have directed initiation ceremonies into war bands, Anthony says.